Here is an excellent paper from Richard Henley et al that provides support for the argument that potassic alteration is largely isochemcial and and not an introduced component. Henley has elequently defined the porphyry copper environment as that of a dynamic, internally and externally stressed, gas phase reactor where repetitive fracturing generates high permeability flow paths for expansion of the magmatic gas phase from source to surface.
SUMMARY of the Abstract
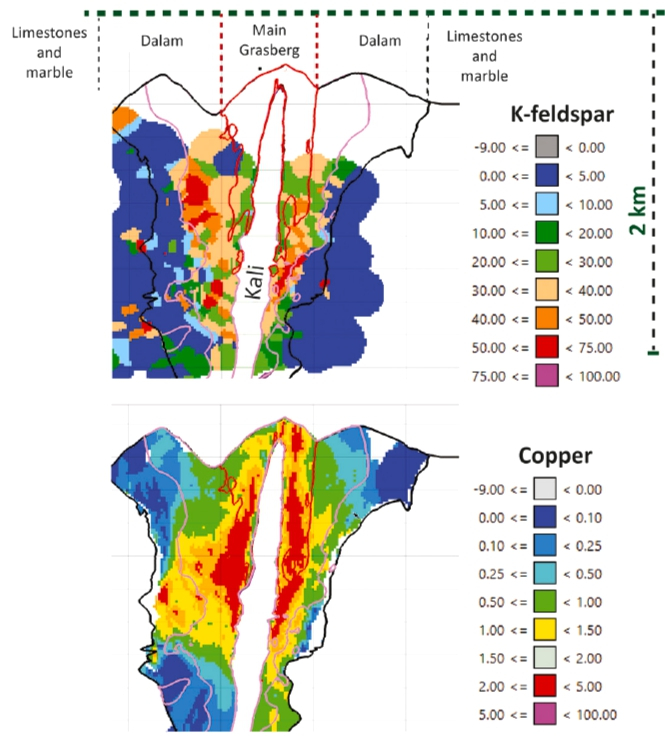
- Potassium silicate alteration is a hallmark of porphyry copper deposits, which provide two thirds of the world’s annual copper demand
- These deposits form in the cores of calc-alkaline to alkaline volcanic systems from the flux of magmatic gas that transports copper and other metals from source to surface
- The Grasberg Cu-Au deposit is a giant deposit that formed within a maar-diatreme complex following a resurgence in magmatism
- The defined resources of this deposit occur from a few hundred meters depth to 1.7 km below the paleosurface, which is partially preserved as a section of maar tuffs
- Potassium silicate alteration is commonly interpreted as the result of the addition of potassium to the host rocks via interaction with a potassium-rich brine of magmatic origin
- Data show that alteration at the deposit scale is isochemical with respect to major rock-forming components and that only sulfur and economic metals are added by flux of reactive magmatic gas
- Silicate solubilities are low, so only a minor fraction of alkalis in the host rock are extracted by alteration reactions and discharged at the paleosurface
- Reaction of magmatic gas phase with plagioclase results in the coupled deposition of anhydrite and disproportionation of SO2 to release H2S
- In-situ release of H2S immediately scavenges Cu and other chalcophile metals from the continuing magmatic gas flux to form the Cu-Fe-sulphides that make up the economic reserve
- Sequestration of Ca into anhydrite and deposition of silica into early quartz veins increases concentration of K2O, Na2O, MgO, etc. in remaining silicate assemblage in porous host rock
- This results in the development of intermingled potassium-enriched silicate and sulphur-rich sub-assemblages that constitute the mineralized phyllic
- Understanding of the alteration processes provides insights into gas-solid reactions processes inside active magmatic arc volcanoes but the magnitude of copper mineralisation is dependent on the original metal content of the source of the magmatic gas phase.
Introduction
Approximately two thirds of the world’s annual copper production is sourced from ‘porphyry copper’ deposits, which are hydrothermal ore deposits that developed in the cores of volcanic systems in magmatic arcs throughout the Phanerozoic. The defining geochemical characteristic of these deposits is the presence of economic grades of sulphide mineralisation (averaging > ~ 0.5wt% copper), along with potassium silicate rock alteration. This alteration is usually divided into ‘potassic’ assemblages (dominated by potassium feldspar and micas) and ‘phyllic’ assemblages (characterised by ‘white’ mica and quartz).
It has long been assumed that the potassium silicate alteration is the result of infiltration of saline liquid-phase brines from intrusives. However, research into the Grasberg porphyry copper-gold deposit has indicated that gas-solid reactions within the sub-volcanic environment likely played a role, with the gas phase composition of high temperature gas mixtures released by fumaroles in modern volcanoes dominating. This raises the question of how such large-scale potassium enrichment occurs without the addition of high salinity brines. To answer this, this paper analyses micro to mine scale petrographic and geochemical data using a range of techniques including high resolution tomography to measure microporosity, based on samples from the Grasberg deposit, which contains over 32 Mt. of copper.
SUMMARY of the Conclusions
- Porphyry copper-gold deposits form within magmatic vapour plumes.
- These plumes contain highly reactive SO2 and HCl, along with Cu and other metals.
- The permeability of these plumes is maintained through their evolution by time variant internal and regional deviatoric stress.
- Porphyry copper deposits are characterized by potassium silicate alteration at the scale of several km3.
- This alteration is directly associated with Cu-Fe-S mineralisation and occurs in large deposits such as the Grasberg deposit.
- Fluid inclusions in such assemblages are commonly assumed to be a potassium-enriched magmatic vapour.
- However, new data from Grasberg show alteration at the deposit scale of several km3 was essentially isochemical for major rock forming elements.
- There is no evidence for the involvement of a K-rich brine or K-enriched magmatic gas phase.
- Apparent potassium enrichment is the result of sequestration of calcium into anhydrite.
- This alteration process continually evolves intergranular porosity during mineral replacement with anhydrite and sulphides.
- This process maintains reactive gas flux in the altering host rock and sustains higher flux of magmatic gas through developing fracture veins.
- A quasi-isochemical hypothesis for potassic alteration in porphyry copper deposits is proposed.
- This alteration process occurs to various extents in evolving magmatic arc volcanoes due to sub-surface gas-solid reactions.
- Porphyry copper formation is correlated with processes inside active volcanoes.
- The relative economic potential of these volcanoes arises primarily from the relative fertility of the magmatic source regime.
